WHAT IS OCEAN ACIDIFICATION ?
Ocean acidification (OA) can be defined as a reduction in the pH of the ocean over an extended period, typically decades or longer, which is caused primarily by uptake of carbon dioxide (CO2) from the atmosphere, but can also be caused by other chemical additions or subtractions from the ocean.
Ocean acidification can also be caused by other (other than carbon dioxide) chemical additions or subtractions from the oceans that are natural (e.g., increased volcanic activity, methane hydrate releases, long-term changes in net respiration) or human-induced (e.g., release of nitrogen and sulphur compounds into the atmosphere).
ANTHROPOGENIC OCEAN ACIDIFICATION
• Anthropogenic ocean acidification refers to the component of pH reduction that is caused by human activity.
• Because human activities are releasing carbon dioxide into the atmosphere very quickly, the ocean is taking up carbon dioxide faster today than it has in the past. This is causing global ocean chemistry to change more quickly than ocean systems can handle.
Must read: CONSEQUENCES OF CLIMATE CHANGE – WORLD
CURRENT STATUS OF OCEAN ACIDIFICATION
Average global surface ocean pH has already fallen from a pre-industrial value of 8.21 to 8.10, corresponding to an increase in acidity of 28.8%. Values of 7.8–7.9 are expected by 2100, representing a 100–150% increase in acidity.
WHY THERE IS A CONCERN ABOUT SUCH A SEEMINGLY SMALL CHANGE IN OCEAN pH ?
• Many organisms are very sensitive to seemingly small changes in pH.
• For example, in humans, arterial blood pH normally falls within the range 7.35–7.45. A drop of 0.2-0.3 pH units in human blood pH can result in rather profound health consequences, including seizures, heart arrhythmia, or even coma (a process called “acidosis”).
• Similarly, many marine organisms are very sensitive to either direct or indirect effects of the change in acidity (or H+ concentration) in the marine environment.
• Fundamental physiological processes such as respiration, calcification (shell/skeleton building), photosynthesis, and reproduction respond to the magnitude of changes in carbon dioxide concentrations in seawater, along with the resultant changes in pH and carbonate ion concentrations that are expected over the next century.
MECHANISM OF OCEAN ACIDIFICATION
As carbon dioxide (CO2) dissolves into seawater,it creates carbonic acid. Through a series of chemical reactions, carbonic acid releases hydrogen ions (H+), which decreases seawater pH, and decreases the concentration of carbonate ions (CO3– –), which provide chemical building blocks for marine organisms’ shells and skeletons.
The detailed process is as follows-
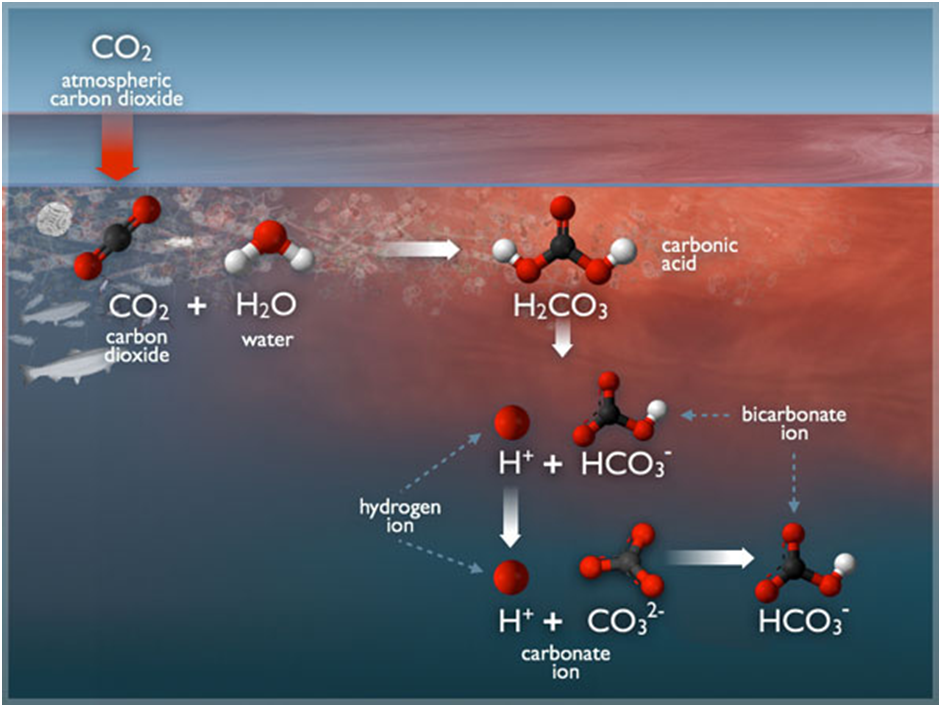
When CO2 dissolves in seawater, it has several consequences for the chemistry of the seawater. Some of the CO2 that dissolves in seawater will remain in the form of a dissolved gas that can freely exchange with the atmosphere and be taken up directly by marine plants and phytoplankton. This fraction is usually referred to as dissolved or aqueous carbon dioxide, and it is typically expressed as the partial pressure of CO2 , abbreviated pCO2.
Some of the molecules of CO2 dissolved in seawater combine with molecules of water (H2O) to form a weak acid, called carbonic acid (H2CO3) . This reaction can be depicted as:
CO2 + H2O ↔ H2CO3
When acids are dissolved in water, they “dissociate,” which means that they break apart into their constituent ions, in this case a hydrogen ion (H+) and a bicarbonate ion (HCO3-, as is found in baking soda).
H2CO3 ↔ H+ + HCO3–
At typical seawater pH values, some of the hydrogen ions will remain as hydrogen ions, thus increasing the acidity and lowering the pH of the seawater. However, most of the hydrogen ions created through the previous reaction will subsequently combine with carbonate ions to form additional bicarbonate ions, thereby reducing the pool of carbonate ions.
H+ + CO3- – ↔ HCO3-
In summary, the chemical changes in seawater resulting from increased atmospheric CO2 concentrations include increases in the concentrations of dissolved (or aqueous) carbon dioxide, hydrogen ions, and bicarbonate ions, and decreases in the carbonate ion concentration and pH.
IMPACT OF ACIDIFICATION ON MARINE LIFE
Organisms may be affected directly or indirectly by changes in the concentrations of any of the forms of inorganic carbon (CO2, HCO3-, CO3- –) discussed above, as well as by acidity levels-
1 . Ocean acidification reduces calcium carbonate – a mineral through which the shells and skeletons of many shellfish and corals are formed. A reduction in the mineral slows the growth of these marine species, and also makes their shells weaker.The reefs also act as a home to various forms of marine life, and the rising acidity in the oceans can lead to their erosion and extinction, thereby threatening the existence of many other species.
2 . The shortage of calcium carbonate affects the microscopic algae that form the base of the marine food web. The algae uses calcium carbonate to build shells, which helps protect them from predators. A drop in the pH levels disrupts the ability of microscopic algae to build shells, and this can have disastrous effects on the food chain.
3 . Pteropods, or swimming sea snails, serve as a critical link of the marine food chain, being the main food source for whales and other predators. Pteropods will not be able to withstand the rising acidity levels and will eventually dissolve, making survival for larger creatures extremely difficult.
4 . Research shows that brittle stars, who act as important burrowers and as food items for flatfish, could face a severe population decline due to the rise in acidity. The adults might lose muscle mass while regenerating their arms, and most of the larvae won’t survive.
5 . It has been observed that ocean acidification slows down the growth of juvenile sea urchins, and provides them with thinner, smaller, and misshapen shells. Weak shells and abnormal growth may make sea urchins more vulnerable to predators, and even disrupt their mating process, by making the sperm of sea urchins swim more slowly, thereby reducing its chances of finding and fertilizing an egg.
External link: https://archive.ipcc.ch/publications_and_data/ar4/wg1/en/ch5s5-4-2-3.html
External link: https://oceanservice.noaa.gov/facts/acidification.html#:~:text=Ocean%20acidification%20refers%20to%20a,CO2)%20from%20the%20atmosphere.
PRACTICE QUESTIONS
QUES . The acidification of oceans is increasing. Why is this phenomenon a cause of concern? UPSC 2012
1 . The growth and survival of calcareous phytoplankton will be adversely affected.
2 . The growth and survival of coral reefs will be adversely affected.
3 . The survival of some animals that have phytoplanktonic larvae will be adversely affected.
4 . The cloud seeding and formation of clouds will be adversely affected.
Which of the statements given above is /are correct?
(a) 1, 2 and 3 only
(b) 2 only
(c) 1 and 3 only
(d) 1, 2, 3 and 4
(a)
QUES . Which among the following can contribute to Ocean Acidification –
1 . Increased volcanic activity
2 . Methane hydrate releases
3 . Release of nitrogen and sulphur compounds into the atmosphere
Codes:
a . 1 & 2
b . 1 & 3
c . 2 & 3
d . 1 , 2 & 3
(d)

Glorious
Mechanism explained very simply.
Great!
Thank you sir.
We need to practice more such questions.