OZONE DEPLETING SUBSTANCES (ODS)
Chlorofluorocarbons, methane, nitrous oxides (N2O), carbon tetrachloride (CCl4), methyl bromide (a soil fumigant and insecticide), aircraft emissions, n- propyl bromide and Halon- 1202 are major agents that cause depletion of ozone layer. Hence, these are called as Ozone Depleting Substances (ODS).
What are Chlorofluorocarbons (CFC) ?
Chlorofluorocarbons are a group of aliphatic organic compounds.
These are a family of synthetic chemicals that are mostly the compounds of chlorine, fluorine and carbon.
These are stable,nonflammable, non-corrosive chemicals with a peculiar trade name Freon. This trade name has been registered by the E.I. du Pont de Nemours & Company.
Being relatively non- toxic chemicals, these are easy and inexpensive to produce.
These were first developed in 1930s but found widespread use only in the years following World War II.
During 1970s CFCs were linked to destruction of ozone layer due to which its manufacture has been banned in most of the countries of the world.
Some important members of CFC group are dichlorodifluoromethane (Freon-12), trichlorofluoromethane
(Freon- 11), chlorodifluoromethane (Freon- 22), dichlorotetrafluoroethane (Freon- 114) and trichlorotrifluoroethane (Freon- 113).
On earth these chemicals are used extensively as aerosol spray propellants, refrigerants, solvents and foam blowing agents.
Human emissions of chlorofluorocarbons (CFCs) and halons (bromine-containing gases) have occurred mainly in the Northern Hemisphere. About 90% have been released in the latitudes corresponding to Europe, Russia, Japan, and North America.
MECHANISM OF OZONE LAYER DEPLETION
Chlorofluorocarbons or Freons get accumulated in greater amounts at high altitudes and gradually reach to the stratosphere. https://www.epa.gov/ozone-layer-protection/basic-ozone-layer-science#:~:text=II.-,Ozone%20Depletion,than%20it%20is%20naturally%20created.
Under the influence of intense short wave ultraviolet radiations they release chlorine atoms.
CFCl3 + electromagnetic radiation → Cl + CFCl2
Ozone is a highly reactive molecule that easily reduces to the more stable oxygen form with the assistance of a catalyst.
Cl and Br atoms destroy ozone molecules through a variety of catalytic cycles.
A chlorine atom reacts with an ozone molecule (O3), taking an oxygen atom to form chlorine monoxide (ClO) and leaving an oxygen molecule (O2).
Cl + O3 → ClO + O2
The ClO can react with a second molecule of ozone, releasing the chlorine atom and yielding two molecules of oxygen.
ClO + O3 → Cl + 2O2
A single chlorine atom can react with more than, 100,000 molecules of ozone and can convert them into oxygen.
Other ozone depleting substances like methane, nitrous oxide, methyl bromide etc. too, pass through a series of reactions under the influence of UV-radiations of sunlight and catalysts found in the air and help in the depletion of ozone layer.https://fotisedu.com/ozone-layer-its-formation-and-significance/
OZONE HOLE
The hole in the context of ozone depletion relates to thinning of the ozone layer in a certain area.
The ozone hole has been recorded both in the Northern and Southern Latitudes .
The ozone hole over Antarctica may expose not only the Antarctica but also a large area of the pacific and Atlantic oceans and South America as well.
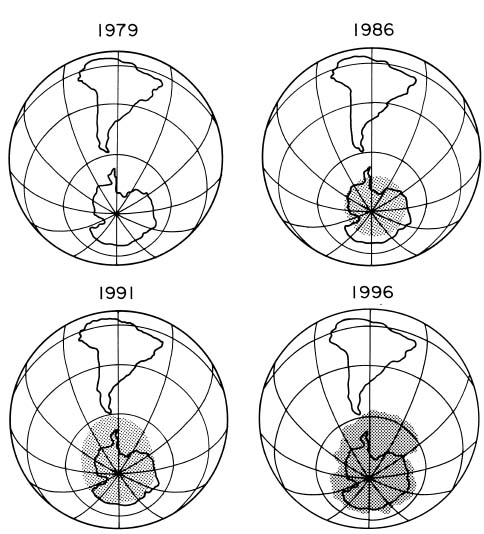
Growth of the Antarctic ozone hole
How the global stratosphere contains nearly equal amounts of chlorine ?
Gases such as CFCs and halons, which are insoluble in water and relatively unreactive, are mixed within a year or two throughout the lower atmosphere.
The CFCs and halons in this well-mixed air rise from the lower atmosphere into the stratosphere mainly in tropical latitudes.
Winds then move this air poleward both north and south from the tropics, so that air throughout the global stratosphere contains nearly equal amounts of chlorine (and bromine).
Why there is correspondingly less ozone depletion in the Arctic than Antarctic?
In the Southern Hemisphere, the South Pole is part of avery large land mass (Antarctica) that is completely surrounded by ocean.
This symmetry is reflected in the meteorological conditions that allow the formation in winter of a very cold region in the stratosphere over the Antarctic continent, isolated by a band of strong winds circulating around the edge of that region.
The very low stratospheric temperatures lead to the formation of clouds (polar stratospheric clouds) that are responsible for chemical changes that promote production of chemically active chlorine and bromine.
This chlorine and bromine activation then leads to rapid ozone loss when sunlight returns to Antarctica in September and October of each year, which then results in the Antarctic ozone hole.
The magnitude of the ozone loss has generally grown through the 1980s as the amount of human-produced ozone-depleting compounds has grown in the atmosphere.
Similar conditions do not exist over the Arctic. The wintertime temperatures in the Arctic stratosphere are not persistently low for as many weeks as over Antarctica, which results in correspondingly less ozone depletion in the Arctic
PREVENTION AND CONTROL OF OZONE DEPLETION
Banning the production and use of ozone depleting substances is one important way of preventing further depletion of the ozone layer in the stratosphere.
On the other hand, alternatives to these chemical compounds should also be searched out so as to replace these chemicals.
Scientists of the University of California, U.S.A. devised a possible way of plugging the ozone hole by injecting alkanes or propanes into the atmosphere of Antarctica. The alkanes have the affinity of reacting with ozone destroying chlorine atoms.
According to the scientists, about 50,000 tonnes of alkane or propane would have to be blown to check the ozone loss. These chemicals could be released from an altitude of about 15 km by a group of hundreds of large aircrafts.
GLOBAL EFFORTS OF PREVENTION
Since ozone depletion is a Global Environmental Problem, it requires strong global efforts and co-operations for its solution.
The International Community is taking up strong efforts as a result of which global consumption of ozone depleting substances has decreased markedly.
Following the UNEP’s Governing Council’s meeting to co- ordinate activities on protecting ozone layer in 1975, United States, Canada, Norway and Sweden banned the use of CFCs. The production capacity of the European Union (E U) was frozen allowing limited uses of aerosols.
In March 1985, 28 countries of the world agreed on Vienna Convention for the protection of the ozone
layer. https://ozone.unep.org/treaties/vienna-convention
In September 1987, different countries of the world adopted Montreal Protocol on substances that deplete ozone layer. https://www.unep.org/ozonaction/who-we-are/about-montreal-protocol
Till now 198 countries ratified the Vienna Convention and 198 the Montreal Protocol.
PRACTICE QUESTIONS
QUES 1. Consider the following statements: Chlorofluorocarbons, known as ozone- depleting substances, are used: UPSC 2012
1 . in the production of plastic foams
2 . in the production of tubeless tyres
3 . in cleaning certain electronic components
4 . as pressurizing agents in aerosol cans
Which of the statements given above is/are correct?
(a) 1, 2 and 3 only
(b) 4 only
(c) 1, 3 and 4 only
(d) 1, 2, 3 and 4
Ans (c) EXPLANATION: ֍ Chlorofluorocarbons are used in the manufacture of aerosol sprays, blowing agents for foams and packing materials, as solvents, and as refrigerants. ֍ Butyl rubber, a copolymer of isobutylene (98%) and isoprene (2%), is almost exclusively used as the inner liner for tubeless tyres and in tyre tubes because of its very low air permeability compared to natural or other synthetic rubbers. For use in inner liners, the butyl is modified through halogenation with chlorine and bromine to improve its compatibility and ensure covulcanization with the other elastomeric materials.

Fantastic information 👍👍👍👍👍
Excellent 👌👌
Ozone hole very well explained