What is Ozone?
Ozone is one of the three allotropes of oxygen, an element in gaseous form. It is triatomic and less stable than oxygen. Its chemical formula is O3.
Must read: DEPLETION OF OZONE LAYER – PREVENTION AND CONTROL
FORMATION OF OZONE LAYER
Ozone in the stratosphere is very important to life. It is formed by the action of the ultraviolet light from the sun on molecules of oxygen.
However, it is mainly produced from oxygen containing molecules such as SO2, NO2, aldehyde etc. also when these molecules are exposed to ultraviolet radiations.
Here is an example of the chemical reaction that takes place during the formation of ozone from NO2.
Firstly NO2 molecule in presence of ultraviolet radiation gets changed to excited molecule of NO2.
NO2 → NO2 (excited molecule)
Then NO2 dissociates to form O(atomic oxygen) & NO
NO2 → O (atomic oxygen) + NO
Then O (atomic oxygen) combines with O2 to form O3
Formation of Ground level ozone
The most significant things that cause ground-level ozone to form are:
NOX and VOCs (from mobile source emissions and industrial processes ), and UV radiation (from sunlight).
We see higher ground-level ozone amounts most often in summer, due to increased amounts of UV radiation during the longer days, but ozone can still form in spring, fall, and even winter given the right conditions.

Even though the emission sources that contribute to ground-level ozone are typically found in urban areas, strong winds can also move it into rural areas, causing them to have high amounts of ground-level ozone.
Where is ozone layer present and what is its thickness?
A large number of ozone molecules assemble around the earth to form the Ozone Layer which extends from 12 to 45 km above the earth surface. On an average it is about 230 Dobson units (DU) in thickness.
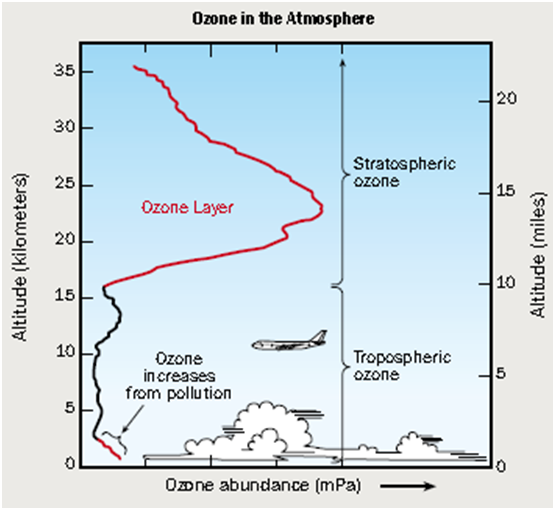
What is Dobson unit?
DU is the unit which measures thickness of the ozone layer. It equals to 0.01 mm.
One Dobson unit is the number of molecules of ozone that would be needed to create a layer of pure ozone 0.01 mm thick at a temperature of zero degrees Celsius at pressure of 1 Atmosphere.
1DU = 2.69 × 1016 ozone molecules.
SIGNIFICANCE OF OZONE LAYER
Ozone absorbs ultraviolet radiations so that much of it is never allowed to reach to the earth
surface.
The protective umbrella of ozone layer in the stratosphere protects the earth from harmful ultraviolet radiations.
Ozone plays an important role in the biology and climatology on the earth’s environment.
It filters out all the radiations that remain below 3000Å. Radiations below this wavelength are biologically harmful. Hence any depletion of ozone layer is sure to exert catastrophic impacts on life in the biosphere.
Ozone is “Good Up High, Bad Nearby”
Ground level ozone is bad ozone. Ozone harms human health and the environment when it forms close to the ground.
People with respiratory conditions such as asthma, or those who are active outside on days when ozone amounts are high can feel shortness of breath, wheezing, and coughing.
ULTRAVIOLET RADIATIONS
There are three types of ultraviolet radiations in the sunlight- ultraviolet-A, ultraviolet-B and
ultraviolet-C radiations.
The UV-A is a low energy radiation with wavelengths 400 to 315 nm (1nm=10Å). It is not harmful to life.
UV- B radiations that comprise 1- 5 percent of the total radiation, is a short wave radiation (315 to 280 nm) with high energy. It is harmful to life.
The UV- C radiation is a radiation of shortest wavelength (280 to 100 nm) with highest quantum of energy. It has great power to damage life but the ozone layer does not allow it at all to pass through and to reach to the earth.
HARMFUL IMPACTS OF ULTRAVIOLET RADIATIONS
(1) UV radiation causes sun- eye- diseases (cataract), skin diseases, skin cancer and damage to immune system in our body.
(2) It damages plants and causes reduction in crop productivity.
(3) It damages embryos of fish, shrimps, crabs and amphibians. The population of salamanders is reducing due to UV-radiations reaching to the earth.
(4) UV- radiations damage fabrics, pipes, paints, and other non-living materials on this earth.
(5) It contributes in the Global Warming. If the ozone depletion continues, the temperature around the world may rise even up to 5.5 Celsius degrees.

Very informative 👍👍
Very good 👍👍
Good job