WHAT IS GENE THERAPY?
Gene Therapy may be defined as a technique in which a patient (sufferer) is given healthy genes to replace the defective ones inherited from the parents, or to enhance the action/reaction of the genes they already have.
Most of the genetic disorders may result in serious complications, health problems and untimely death. Techniques are being developed to replace defective genes or manipulate them to remove the genetic disorder. Such treatment is called Gene Therapy.
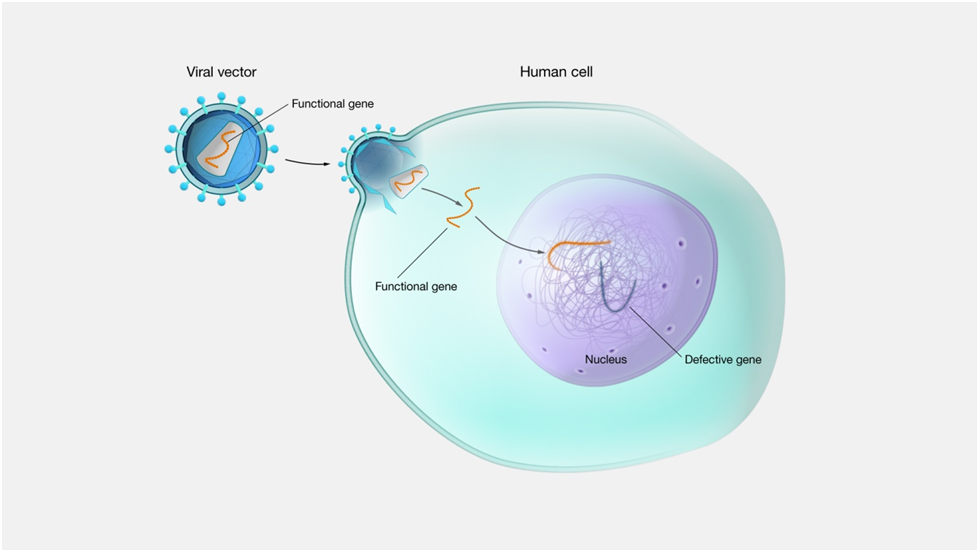
TYPES OF GENE THERAPY
There are two basic types of human gene therapy:
(i) Somatic gene therapy, and
(ii) Germ-line gene therapy.
(i) Somatic (body cell) gene therapy
Once a normal gene has been cloned, it can be used to correct a genetic defect. Body cells are targeted for genetic transformation (defective gene transformed to normal). This approach helps in the correction of a genetic defect confined to a specific organ or tissue.
(ii) Germ line (sex cell) gene therapy
In this approach, cells of germinal epithelium or gametes or zygote are genetically modified to create an individual that will carry remedial gene(s) in the following generation.
PRESENT RESEARCH (SOMATIC GENE THERAPY )
Presently all research on human gene therapy is directed towards correcting gene defects in somatic cells (non-sex cells). Somatic gene therapy can be grouped under the broad categories of :
(a) Ex-vivo gene therapy,
(b) In-vivo gene therapy, and
(c) Antisense gene therapy.
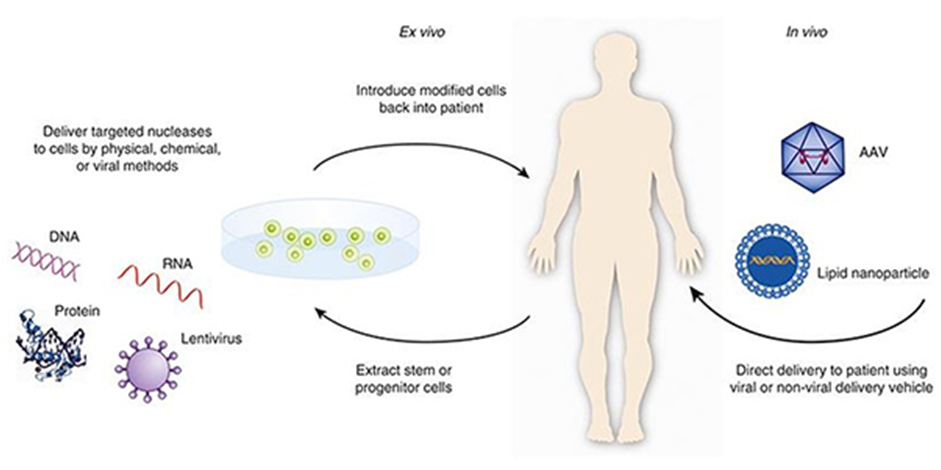
(a) Ex-vivo (outside the body) gene therapy:
This type of therapy usually involves the use of cells (with defective gene) taken from the patient. After the gene alteration when the same cells are transfused (transferred back), no immunological response takes place. The steps involved in the procedure are :
1 . Isolating the cells with gene defects from a patient.
2 . Growing the isolated cells in culture.
3 . Altering the genome of the isolated cells with remedial gene.
4 . Selecting, growing and testing the altered cells.
5 . Transplanting or transfusing the altered cells back into the patient .
Vectors such as retrovirus is used for the integration of normal gene in the host genome. Stem cells of the bone marrow are continuously producing new cells. If such cells are taken and put back after alteration, to remove genetic defects, these cells can divide and differentiate into various important cells such as B cells and T cells, macrophages, red blood cells, platelets and bone cells.
Genetically engineered stem cells on transplanting back into the patient’s body result in a continuous supply of the required gene product. The technique can be used in the treatment of the following genetic disorders:
(i) Severe Combined Immuno Deficiency (SCID).
(ii) Sickle cell anaemia.
(iii) Thalassaemia
(iv) Certain tumours.
(b) In-vivo (within the body) gene therapy
This type of gene therapy includes direct delivery of a remedial gene into the cells of a particular tissue of the patient. Adenovirus, a double stranded DNA virus, is being used as a vehicle for transferring the remedial gene. The viruses used are weak enough to cause any disease. The tissue specific virus integrates with the host genome and can only infect dividing cells and not the other healthy cells.
This therapy may become useful in the treatment of cancer, Alzheimer’s disease and Parkinsons’s disease.
(c) Antisense Therapy
This therapy is designed to prevent or lower the expression of specific gene thus limiting the amount of translation of protein from the over producing gene.This therapy involves the introduction of nucleic acid sequence that is complementary to all or part of m-RNA (messenger RNA formed in the target cell) into the cells overproducing the gene product .
This therapy will prove useful in certain human genetic diseases and cancers where too much of a gene product or its continuous presence changes the normal functioning of the cell. It has been tried for treatment of malignant glioma or brain tumour.
LIMITATIONS OF GENE THERAPY
Gene therapy has the following limitations:
(i) Research is limited to only somatic cells. Treated individuals can not pass the genetic improvement to offspring.
(ii) There could be a possibility of random integration of DNA into a human chromosome leading to inactivation or activation of a normal gene. This may result in either deficiency of an important enzyme or uncontrolled cell division leading to cancerous growth.
(iii) The Procedure Planned has to meet strict safety standards in animal trials.
(iv) Target diseases have to be limited to those that involve known defects in a single gene, and the normal gene must be cloned and be available for transplant.
