Seawater has many different gases dissolved in it, especially nitrogen, oxygen and carbon dioxide. Each and every day, there is a continuous and dynamic exchange of these gases and water between the atmosphere and the oceans. This exchange is helped by the mixing of the surface by wind and waves.
Ocean-atmosphere exchange affects the lives of animals, plants, and microbes that live both on land and in the oceans.
Oxygen Production on Earth
Roughly 72% of the oxygen production on Earth comes from the plant life in the ocean. The majority of this production is from oceanic plankton ( nearly 50%) — drifting plants, algae, and some bacteria that can photosynthesize. One particular species, Prochlorococcus, is the smallest photosynthetic organism on Earth. But this little bacteria produces up to 20% of the oxygen in our entire biosphere. That’s a higher percentage than all of the tropical rainforests on land combined.
It’s important to remember that although the ocean produces 72% of the oxygen on Earth, roughly the same amount is consumed by marine life. Like animals on land, marine animals use oxygen to breathe, and both plants and animals use oxygen for cellular respiration. Oxygen is also consumed when dead plants and animals decay in the ocean.
Trees and rainforests produce approximately 28% of the oxygen on Earth. Until recently it was thought that the trees in the Amazon rainforest alone produced 20% of the Earth’s oxygen; something that had to be thoroughly reconsidered taking into account the level of oxygen produced by marine plants. Climate scientists have since estimated a more reasonable 6–9% oxygen produced by Amazon rainforest.
Production and Consumption of Oxygen and Carbon Dioxide in Oceans
Oxygen and carbon dioxide play important roles in the lives of marine organisms. Bacteria, phytoplankton, algae, and plants use carbon dioxide in the process of photosynthesis, producing oxygen as a by-product.
Most living organisms require oxygen and produce carbon dioxide. The process of decomposition, which requires respiration by bacteria or other organisms, also uses oxygen and produces carbon dioxide.
Oxygen as a by-product of photosynthesis added to seawater only near the surface. Carbon dioxide, on the other hand, is added at all depths by the processes of decomposition and respiration. The levels of these two gases in seawater have a profound effect on the kinds of organisms that can live in a given area and the number if organisms a particular region can support.
Variation in Oxygen Content with Depth in Oceans and Seas
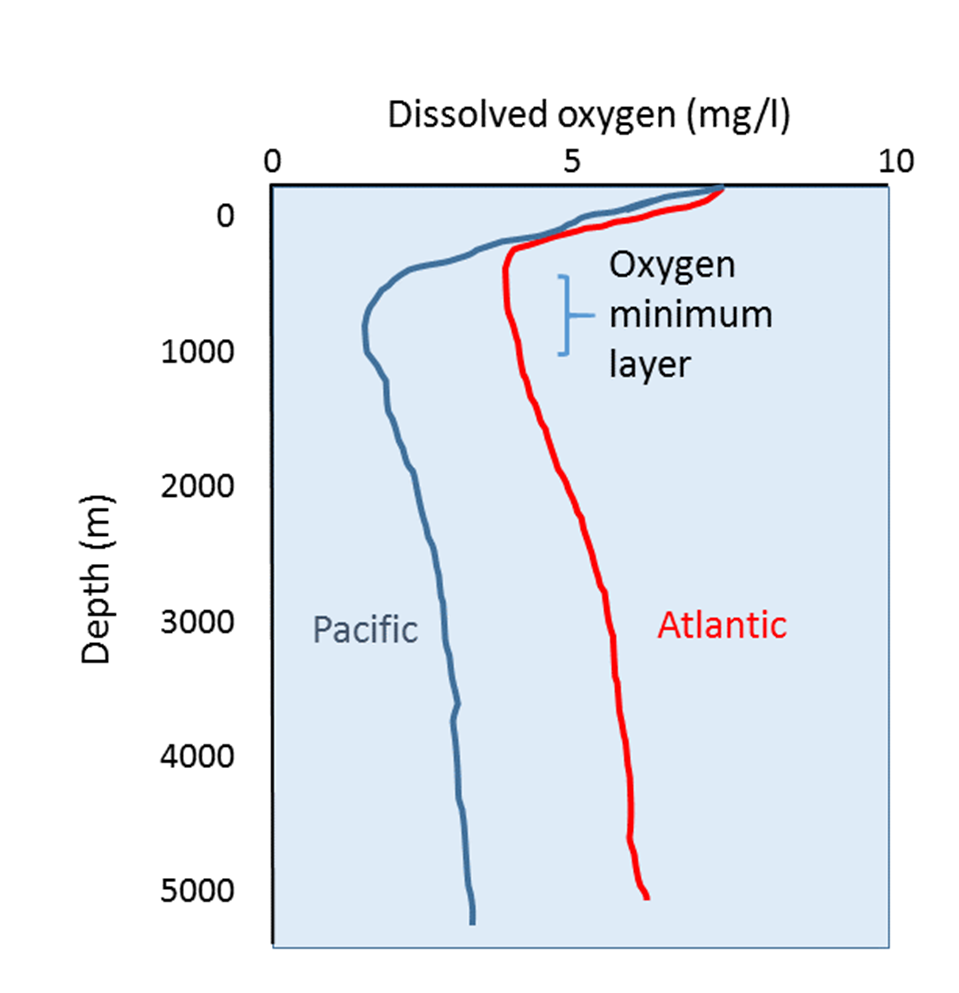
Oxygen content is highest at the surface of oceans for two main reasons:
1 . This is where oxygen dissolves into the ocean from the atmosphere.
2 . The surface water is where oxygen is produced by phytoplankton through photosynthesis.
Respiration is also occurring in the surface waters, but the rate of photosynthetic oxygen production is greater than the rate of removal through respiration.
It should be noted that even though dissolved oxygen is highest at the surface, there is still far less oxygen in the water than is found in the air. Well-oxygenated surface water may only contain around 8 mg O2/l, while the air contains 210 mg O2/l.
As depth increases, dissolved oxygen declines, reaching a minimum between a few hundred meters and 1000 m deep, the aptly-named oxygen minimum layer. At these depths and below, the water is too far removed from the surface for any atmospheric exchange, and there is not enough light to support photosynthesis, so there is little if any oxygen added to the water. At the same time, oxygen is removed from the water through the respiration of deep water organisms, and the decomposition of organic material by bacteria as it sinks to depth.
Below the oxygen minimum layer there is often an increase in dissolved oxygen at the greatest depths. This bottom water is usually colder than the surface water and is under enormous pressure. Lower temperatures and higher pressure increase the solubility of dissolved gases.
But there is another reason that bottom water contains more oxygen than mid-water depths that has to do with the way water circulates throughout the deep ocean. In polar regions, the cold surface water absorbs lots of oxygen. This cold, oxygen-rich water sinks to the bottom due to its high density, taking the oxygen with it. The oxygen-rich bottom water will then spend the next thousand years or so moving over the seafloor throughout the major ocean basins. This deep water circulation is the source of oxygen for bottom-dwelling (benthic) organisms.
The oxygen-rich bottom water forms in the polar regions of the Atlantic, and slowly makes its way to the Pacific, with oxygen being removed for respiration along the way. This is why dissolved oxygen levels in Pacific deep water are generally lower than in the Atlantic.
Factors which determine percentage of dissolved oxygen in oceanic water
The amount of gas that water can hold depends on the temperature, salinity, and pressure of the water. For instance, cold water holds more gas than warm water, and more gas will dissolve if the salinity of the water is low and the gas pressure is high.
What are Hypoxic Zones in Oceans?
Areas where dissolved oxygen levels are too low to support most life are referred to as hypoxic zones (they are experiencing hypoxia, or low oxygen). Hypoxia is usually defined as oxygen levels below 2 mg/L. Anoxic zones (anoxia = without oxygen) show more severe forms of hypoxia, with oxygen below 0.5 mg/L.
Must read: Consequences of spreading of ‘Dead Zones’ on marine ecosystem
Some parts of the oceans may experience seasonal or temporary periods of hypoxia, while in other areas these conditions may last much longer. These hypoxic conditions often lead to mass die-offs of marine organisms who struggle to survive without sufficient oxygen.
PRACTICE QUESTIONS
QUES . With reference to the planet Earth, consider the following statements: UPSC PRELIMS 2025
I. Rain forests produce more oxygen than that produced by oceans.
II. Marine phytoplankton and photo-synthetic bacteria produce about 50% of world’s oxygen.
III. Well-oxygenated surface water contains several folds higher oxygen than that in atmospheric air.
Which of the statements given above is/are correct?
(a) I and II
(b) II only
(c) I and III
(d) None of the above statements is correct
Answer – (b)
