Direct air capture technology is a form of carbon dioxide (CO2) removal that takes CO2 from ambient or still air. The separated CO2 can then be permanently stored deep underground, or it can be converted into products.
If the extracted CO2 is sequestered in safe long-term storage, the overall process is called direct air carbon capture and sequestration (DACCS), achieving carbon dioxide removal. Systems that engage in such a process are referred to as negative emissions technologies (NET).
DAC was suggested in 1999 and is still in development. Several commercial plants are planned or in operation in Europe and the US. Large-scale DAC deployment may be accelerated when connected with economical applications or policy incentives.
Direct Air Capture (DAC) vs Carbon Capture and Storage (CCS)
Direct air capture (DAC) technologies extract CO2 directly from the atmosphere at any location, unlike carbon capture and storage (CCS), which captures CO2 from point sources (at the point of emissions) such as a steel plant, a cement factory or a bioenergy plant. Thus, DAC can be used to capture emissions that originated in non-stationary sources such as airplanes.
Significance of Direct Air Capture (DAC) Technology
Carbon dioxide removal is increasingly important because CO2 emissions are warming the Earth’s climate in ways not seen in millions of years. These emissions are causing wildfires, floods, and storms. They are also threatening sea life due to increased acidity in the oceans.
Direct air capture has a critical role in helping the world address legacy emissions and is a key approach needed to achieve a net-zero emissions future.
DAC is a carbon negative technology. Proponents of DAC argue that it is an essential component of climate change mitigation.
Researchers posit that DAC could help contribute to the goals of the Paris Agreement (namely limiting the increase in global average temperature to well below 2 °C above pre-industrial levels).
Approaches for Direct Air Capture
Scientists use two broad approaches for direct air capture: liquid solvents and solid sorbents.
Solvent-based direct air capture systems pass air through chemicals that remove the CO2. Existing systems use a combination of heat and vacuum to remove the captured CO2 and return the chemicals to the direct air capture process. The system then returns the treated air — now with less CO2 — to the atmosphere.
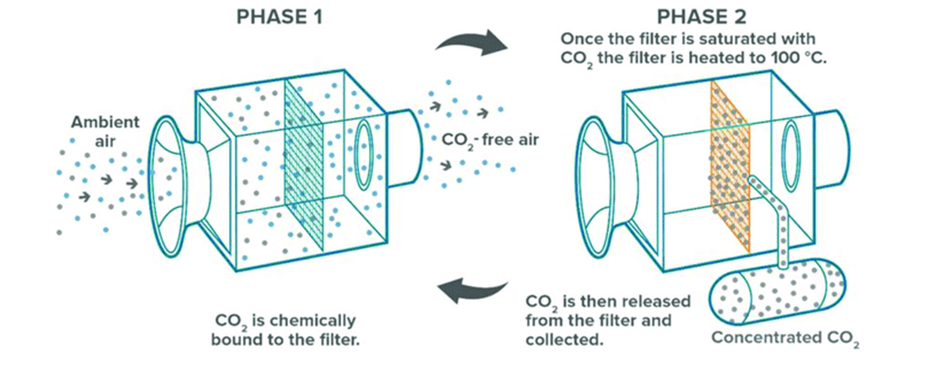
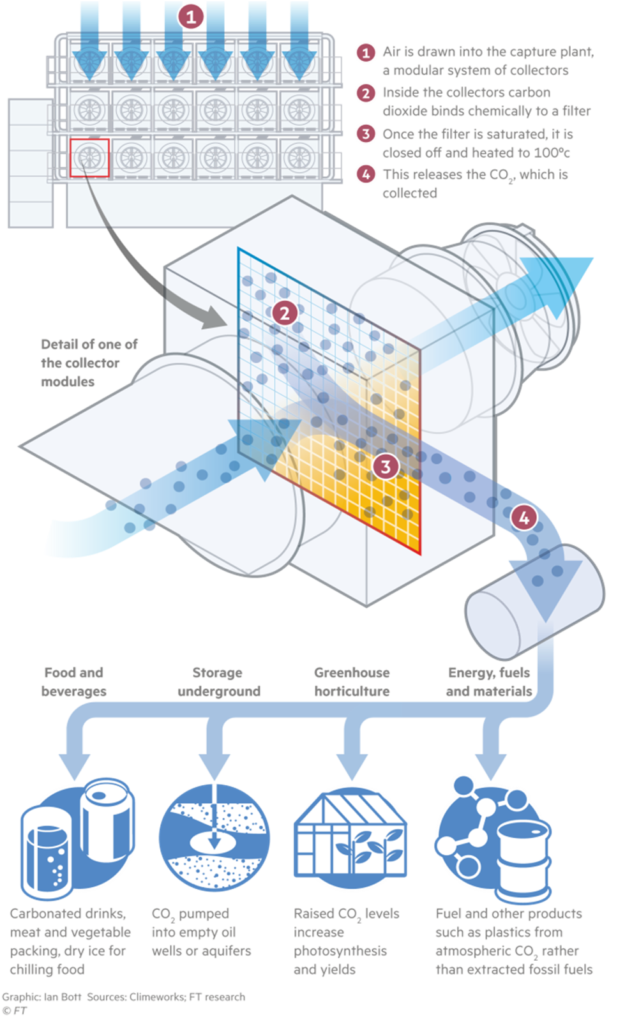
Sorbent-based direct air capture systems use physical filters. These filters chemically bind with CO2 molecules. When the filters are heated and/or placed under a vacuum, they release the CO2, which is now in a concentrated stream. This concentrated CO2 stream is either stored in geologic formations or used. For example, researchers are working on approaches for converting CO2 into building materials, chemicals, and fuels.
Challenges in Direct Air Capture Technology
One of the largest hurdles to implementing DAC is the cost of separating CO2 from air. Although DAC implementation was initially and optimistically estimated to cost around $100–300 per tonne, as of 2023 it is estimated that the total system cost is over $1,000 per tonne of CO2. Thus, opponents of DAC argue that the resources required to operate DAC technologies, are an immense burden that may outweigh the goal of the technology itself.
Some DAC technologies, especially liquid systems, require both high temperature heat and electricity. In these systems the electrical demand is made using natural gas, imported electricity from the grid, and oxyfuel combustion of natural gas. This means that many DAC technologies are powered by fossil fuels, the very thing the technology is meant to eliminate reliance on. However, still DAC is a carbon negative technology, with its greenhouse gas emissions (GHG) estimated to range from 0.01 tCO2 emitted per tCO2 captured when renewable electricity is used to 0.65 tCO2 emitted per tCO2 captured when grid electricity and natural gas (NG) heating are used. Thus, the aim of DAC of offsetting emissions could still be achieved.
DAC relying on amine-based absorption demands significant water input. It was estimated, that to capture 3.3 gigatonnes of CO2 a year would require 300 km3 of water, or 4% of the water used for irrigation. On the other hand, using sodium hydroxide needs far less water, but the substance itself is highly caustic and dangerous.
Individuals concerned with protecting animal life also argue that increasing demand for land for Direct Air Capture would be an additional threat to biodiversity. Opponents also argue that the risk of a Carbon Dioxide leak outweighs the potential benefits.
Applications of Direct Air Capture Technology
Practical applications of DAC include:
Enhanced oil recovery.
Production of carbon-neutral synthetic fuel and plastics.
Beverage carbonation.
Carbon sequestration.
Improving concrete strength.
Creating carbon-neutral concrete alternative.
Enhancing productivity of algae farms.
Enrichment of air in greenhouses.
Liquid fuels.
Enhanced coal bed methane.
PRACTICE QUESTIONS
QUES . With reference to ‘Direct Air Capture’, an emerging technology, which of the following statements is/are correct? UPSC PRELIMS 2025
I. It can be used as a way of carbon sequestration.
II. It can be a valuable approach for plastic production and in food processing.
III. In aviation, it can be a source of carbon for combining with hydrogen to create synthetic low-carbon fuel.
Select the correct answer using the code given below.
(a) I and II only
(b) III only
(c) I, II and III
(d) None of the above statements is correct
Answer – (c)
