Background
Mustard flowers contain both male and female organs and the crop is pre-dominantly self-pollinating. Therefore, a pollination control mechanism is required to disallow self-pollination and encourage cross-pollination for hybrid seed production.
For this, one of the two parental lines of a hybrid has to be made male sterile so that it receives pollen from the other parent to set seed. Seeds harvested from the male sterile line are hybrid seeds which can be provided to the farmers, who can reap the benefit of higher productivity of the hybrids.
How male sterile lines can be developed?
Male sterile lines can be developed by using cytoplasmic male sterility (CMS) through conventional breeding or by genetic engineering using transgenes.
(a) Cytoplasmic male sterility (CMS)
A number of CMS systems have been tested in mustard. However, CMS/ restorer systems have been found to be inadequate for large scale hybrid seed production with high purity. CMS systems are either unstable or their restoration to fertility is inadequate.
(b) Genetic engineering using transgenes
A more versatile hybrid seed production system is based on the use of transgenes – barnase and barstar as explained below.
What is Barnase-barstar system?
The genetically engineered barnase/barstar system provides an efficient and robust alternative method for hybrid seed production in mustard and it has been successfully deployed in countries like Canada, Australia and America for many decades.
A novel way to developing male sterile (MS) lines through genetic engineering was developed by scientists in Belgium in early 1990s through the use of two genes – barnase and barstar from soil
bacterium Bacillus amyloliquefaciens.
In nature, bacterium excretes a defense protein called Barnase (a type of ribonuclease) which degrades the RNA of competing bacteria in an ecological niche. To protect itself from Barnase, the bacterium produces another protein called Barstar which tightly binds with Barnase and renders it ineffective.
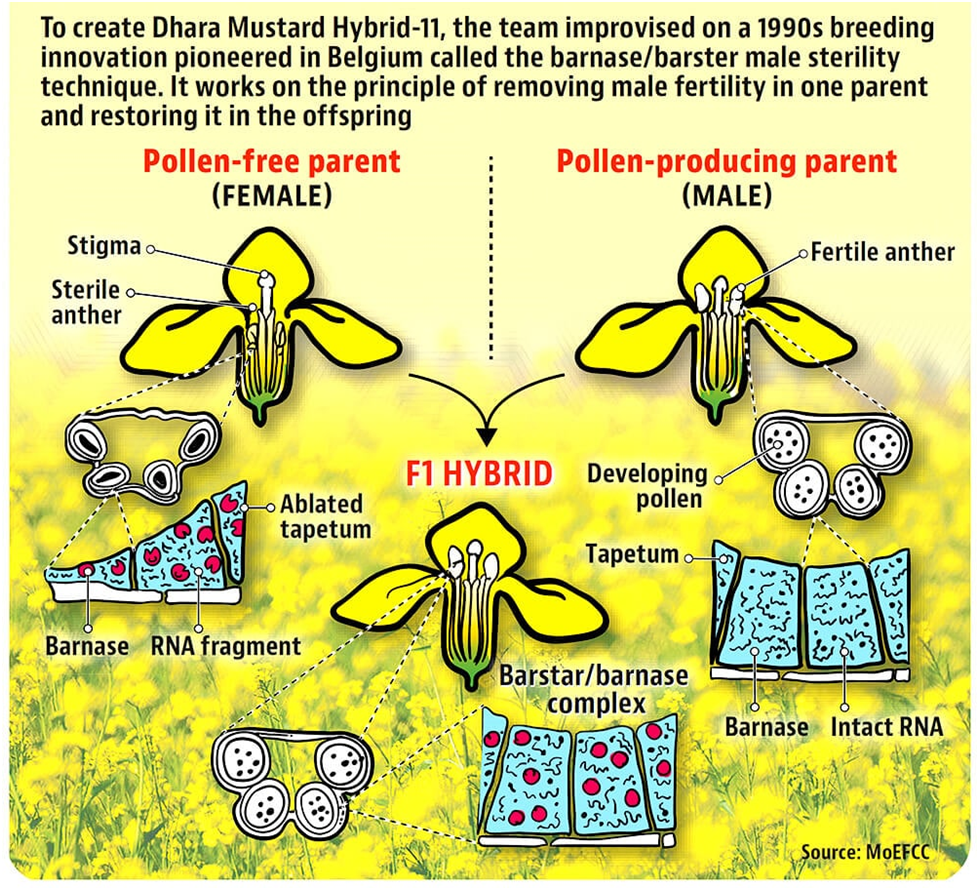
Bacterial genes can only express in plants if these are expressed under plant promoters. Both Barnase and Barstar encoding genes were expressed under a tapetum specific promoter. Tapetum is a layer of cells in the male organs called anthers present in the flower. Tapetum produces metabolites which are essential for the development of mature pollen.
In the barnase gene containing lines, the tapetum tissue ablates (dies),as a consequence developing pollen degenerate, providing MS lines. The other parental line, called restorer of fertility (RF) line, contains barstar gene that also expresses in the tapetum cells.
The MS line receives pollen from the RF line through wind pollination or bee pollination, resulting in the production of hybrid seed that has both the barnase and the barstar genes. When hybrids are grown by the farmer these are fully fertile.
Thus the MS/ RF system ensures that the MS line will only produce hybrid seeds by outcrossing with RF lines thereby providing an efficient system of pollination control for production of hybrid seed. The system hereafter is referred to as barnase-barstar system.
Developer of this new technology in India
In India, the Centre for Genetic Manipulation of Crop Plants (CGMCP), University of Delhi South Campus, New Delhi has made a successful attempt with some alterations in the barnase/ barstar system which culminated in the development of GM mustard hybrid MH11 which has undergone the required regulatory testing processes during 2008-2016.
Issues of concern for the environment
Issues of concern include –
1 . Capability of the genetically engineered plant to escape and potentially introduce the engineered genes into wild populations
2 . Persistence of the gene after the genetically engineered plant has been harvested
3 . Susceptibility of non-target organisms (e.g. insects which are not pests) to the gene product
4 . Stability of the gene
5 . Reduction in the spectrum of other plants including loss of biodiversity
6 . Increased use of chemicals in agriculture.
Are GM foods safe?
Different GM organisms include different genes inserted in different ways. This means that individual GM foods and their safety should be assessed on a case-by-case basis and that it is not possible to make general statements on the safety of all GM foods.
GM foods currently available on the international market have passed safety assessments and are not likely to present risks for human health. In addition, no effects on human health have been shown as a result of the consumption of such foods by the general population in the countries where they have been approved.
Transgenic crops in global perspective
Globally, GM crops are grown on 195 million ha area in more than 30 countries. In several countries, adoption rates of GM traits have been very high; more than 95% in some cases. There is no evidence of adverse effects reported from use of GM crops globally.
Bulk of produce from GM crops like maize, soybean, etc. is exported from USA, Argentina and Brazil, the major GM crops growing countries to many countries including EUs as animal feed and these countries are earning substantial foreign exchange by exporting GM crops.
Current status about introduction of GM mustard
The Government has approved the environmental release of Genetically Modified (GM) Mustard hybrid DMH-11 and its parental lines during 147th meeting of Genetic Engineering Appraisal Committee (GEAC) on 18 October, 2022 for its seed production and testing.
PRACTICE QUESTIONS
QUES . With reference to the Genetically Modified mustard (GM mustard) developed in India, consider the following statements : UPSC 2018
1 . GM mustard has the genes of a soil bacterium that give the plant the property of pest-resistance to a wide variety of pests.
2 . GM mustard has the genes that allow the plant cross-pollination and hybridization.
3 . GM mustard has been developed jointly by the IARI and Punjab Agricultural University.
Which of the statements given above is/are correct ?
(a) 1 and 3 only
(b) 2 only
(c) 2 and 3 only
(d) 1, 2 and 3
(b)
